It is a poisonous liquid that is known to act as a selective fluorinating agent especially in organic synthesis reactions.
Valence Electrons
The valence electrons are the electrons that are farthest from the nucleus of an atom i.e. the electrons that are present in the valence shell of an atom. These electrons actively participate in the chemical bonds that an atom forms with other atoms and are also given away (or taken up) from one atom to another in the case of ionic bonding.
Octet Rule
Noble gases are considered the most stable elements of the periodic table as they do not easily compromise their properties by reacting with other atoms. It is already known that all noble gases, except helium, have eight electrons in their outermost shell. Therefore, it is established that an atom of any element becomes stable when it has eight valence electrons. This is known as the octet rule. As per this rule, all the atoms tend to form chemical bonds with other atoms either by sharing or donating, or accepting electrons from their valence shells in an attempt to complete their octet or acquire eight valence electrons in order to achieve stability.
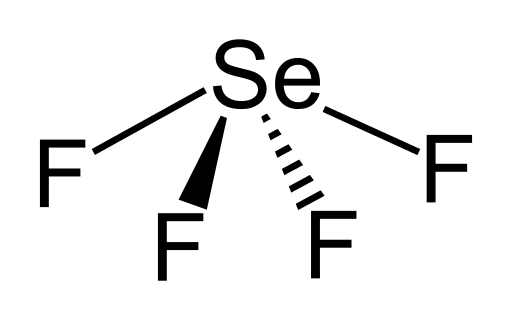
SeF4 Lewis Structure
Lewis structures were introduced by Gilbert N. Lewis in 1916. They introduced the convention of presenting the atoms through their atomic symbols and their valence electrons as the dots surrounding them. Like any other method of representing molecular structures, it aims at establishing the most stable structure for a molecular with formal charge closest to zero and electronic configuration of valence shell closest to 8 i.e. in accordance with octet rule. These are also called Electron dot structures or Lewis dot structures. The Lewis structure for SeF4 is written as:
It can be seen in the structure that the octet for all the five atoms bonded to form the SeF4 molecule is satisfied i.e. the electronic configuration of all the four fluorine atoms as well as the selenium atom is eight.
Steps of Drawing Lewis Structure of SeF4
Let us understand the step by step process for drawing the lewis structure for the SeF4 molecule: • First of all, we will calculate the number of valence electrons for all the atoms present in the molecule. As selenium is a group 16 element it consists of 6 electrons in its valence shell while fluorine is a group 17 element therefore, all the fluorine atoms have 7 valence electrons. Hence, selenium is short of 2 electrons in obtaining its octet while fluorine atoms require one electron each. Now, counting total number of valence electrons for SeF4 molecule: Selenium = 6 Valence electron Fluorine = 7 valence electrons For 4 Fluorine atoms, 7 X 4 = 28 Therefore, total number of valence electrons = 28 + 6 = 34 • In the second step, we will choose a central atom for the molecule. It is assumed that all the other atoms of a molecule are bonded with the central atom. Usually, the least electronegative atom is chosen as the central atom. In the case of selenium tetrafluoride, selenium is less electronegative than fluorine and is, therefore, chosen as the central atom. All the four fluorine atoms are joined to the selenium atom with a single bond.
• The above step helps us calculate the number of electrons further required for the completion of an octet for all the atoms present in the molecule. Every single bond indicates two shared electrons. As fluorine already had 7 valence electrons, the octet for all the four fluorine atoms is completed with the sharing of one electron each from the selenium atom, and selenium which had six valence electrons now has 10 electrons in its valence shell which is rather more than usual. • Here, you may ask how can selenium have 10 valence electrons when the maximum number of electrons allowed in the valence shell of an atom is 8. The answer to this question is the concept called “Expanded Octet”. • Actually, certain elements in the periodic table such as noble gases have the efficiency to expand their octet beyond the normal limit i.e. they are allowed to have more than 8 electrons in their valence shell. The reason for this privilege is the presence of an available d energy sublevel. As selenium is one of those privileged atoms and is, therefore, able to accommodate 10 valence electrons in the SeF4 molecule. • Therefore, the final lewis structure for Selenium Tetrafluoride looks like this:
• To ascertain that the lewis structure drawn is the best possible representation of the structure of that particular molecule we shall calculate the formal charge on the molecule. It is a hypothetical concept which states that for a molecule to have a stable lewis structure it must have the formal charge closest to zero. It is calculated individually for every atom of the molecule. The formula for the formal charge is given as: Formal Charge = [Total no. of valence e– in Free State] – [Total no. of non-bonding pair electrons (lone pair) – 1/2 (Total no. of bonding e–)] Calculating the formal charge for SeF4 molecule: For selenium atom: FC = [6] – [2] – ½ [8] Therefore, Formal Charge on Selenium = 0 Now, for fluorine atom: FC = [7] – [6] – ½ [1] Therefore, FC on Fluorine = 0 Hence, the total formal charge on the molecule is 0 indicating it to be the best possible lewis structure possible. Check out the article on SF4 Lewis Structure.
SeF4 Molecular Geometry
The molecular geometry of a molecule is derived as per the Valence Shell Electron Pair Repulsion (VSEPR) Theory. As per this theory, the electrons inside a molecule tend to arrange themselves as distant from each other as possible in an attempt to avoid the inter-electronic forces. It is assumed that inter-electronic repulsion exists between the electrons of the same atom as well as the electrons of the different atoms and it is strongest between the lone pairs of electrons as they are free to move in space. The difference in the electronegativity of the participating atoms works as the driving force for repulsion. The lewis structure drawn above is the ideal structure for the SeF4 molecule when no repulsion forces are present. However, the ideal situation does not exist in reality due to the various forces that are active inside the molecule and affect its shape and structure. As per the VSEPR theory, the repulsion forces between the bonding as well as non-bonding pairs of electrons determine the actual structure of a molecule. In the case of Selenium tetrafluoride, Selenium is the central atom to which four fluorine atoms are attached through a single bond. Also, one lone pair of electrons is present on the selenium atom. All these electrons push each other as far as possible. The force of repulsion decrease in the order as below: Lone pair-lone pair > Lone pair-bond pair > Bond pair-bond pair Therefore, in the SeF4 molecule, the bond pairs get relatively closer to each other and at the maximum distance possible from the lone pair of electrons resulting in the see-saw shape of the molecule. The electronic geometry for the SeF4 molecule is trigonal bipyramidal and the molecular symmetry is C2V.
This structure has two axial and two equatorial bonds. The bond length for the axial Se-F bond is 177 pm with a 169.2° bond angle between F-Se-F while the bond length for the equatorial Se-F bond is 168 pm with a bond angle of 100.6° between F-Se-F.
SeF4 Hybridization
The hybrid orbitals are formed due to the intermixing of orbitals with similar energy levels. This process is known as hybridization. Linus Pauling in 1931 introduced the concept of hybridization. The hybrid orbital derives their names from the orbital getting mixed for their formation. For example, the name sp for a hybrid orbital indicates that one s and one p orbital were mixed in the hybridization process. The hybridization state for any molecule is determined by calculating the steric number from its bonding and non-bonding electrons per the following formula: Steric No. = Number of sigma (σ) bond on central atom + lone pair on the central atom In the case of Selenium tetrafluoride, No. of sigma bond = 4 No. of lone pair = 1 Therefore, Steric number = 5 The following table describes the hybridization state for a molecule based on their steric number: As the steric number for the SeF4 molecule is 5. Therefore, using the above table, it can be determined that it has a sp3d hybridization state. Let us try to understand the hybridization state for Selenium tetrafluoride. • In normal state Selenium consists of six valence electrons i.e. it has a sp3 hybridization state, which can be represented as follows:
• The valence electrons are distributed amongst the 4s and 4p orbitals while the 4d orbital is completely vacant. • As the Selenium atom gets excited one electron from the 4p orbital jumps into the 4d orbital. However, the 4s orbital remain unaffected as only four fluorine atoms are required to be bonded.
• These four excited unpaired electrons form a sigma bond with the four fluorine atoms while the 2 electrons in the 4s orbital do not participate in bonding. • Therefore, with four sigma bonds and one lone pair of electrons the hybridization for the SeF4 molecule becomes sp3d. • The presence of vacant 4d orbitals allows selenium to have an expanded octet due to which it is able to accommodate more than 8 electrons in its outermost shell.
Polarity of SeF4
The polarity of a molecule arises due to the electronegativity difference between the bonding atoms. It refers to the development of a partial positive charge on one atom and a partial negative charge on another due to the unequal sharing of electrons between these atoms. Usually, a molecule becomes polar if the electronegativity difference between the two bonding atoms is more than 1.5. However, in the case of the uniform geometry of a molecule, the charges cancel out amongst themselves while if the molecular structure is distorted due to uneven distribution of charges a net dipole moment exists on the molecule. In the case of the SeF4 molecule, the electronegativity of the fluorine atom is 4 while that of the selenium atom is 2.4. Hence, the electronegativity difference is 1.6 which is more than 1.5 indicating that partial negative charge develops on fluorine atom while partial positive charge develops on selenium atom. Also, the shape of the SeF4 molecule is distorted further suggesting that the molecule should have a net dipole moment. Read out the article Polarity of SeF4 Polarity of SF4
Properties
A few important properties of Selenium tetrafluoride are given in the table below:
Conclusion
The Lewis structure for Selenium tetrafluoride is written as:
The molecular geometry for SeF4 is a see-saw, electronic geometry is trigonal bipyramidal and the molecular symmetry is C2V. The steric number for the SeF4 molecule is 5 and the hybridization state is sp3d. Due to the electronegativity difference between Selenium and fluorine atoms the SeF4 molecule is polar. Happy learning!!